A tale of two viruses
Synopsis : New and old honey bee viruses. Are these a cause for concern in our bees, or for bees they share the environment with? Facts, suspicions, omissions and reality.
Introduction
A tale of two bee viruses.
One new virus that you probably have not heard about but might be concerned about once you know a little more … but probably shouldn’t be.
And another virus that you do know about and which some non-beekeepers are concerned about … but possibly shouldn’t be.
Inevitably this is going to involve a bit of science, but I’ll try and restrict it to the minimum needed to get the message across.
Buckle up!
Virology 101
A virus is an obligate intracellular parasite.
They are parasites because they lack some of the ‘machinery’ necessary for living and reproducing, so they ‘steal’ these functions from another organism – the host.
Intracellular because they can only steal these functions once inside the host … and not just wandering around in the blood or the guts, they have to be inside the cells of the host.
Finally, obligate {{1}} because this is the only way that a virus can replicate.
Replication is a virologists term for reproduction. It means both making more of and copying, because the act of reproduction involves a single virus making tens, hundreds or thousands of copies of itself.
Because the virus replicates within the host cells and steals some of the functions of the cell to help this process, it can result in cell damage or death.
This may or may not damage the host.
A typical human contains about 30 trillion cells {{2}}. The loss of a few hundred or hundreds of thousands to virus replication may well go unnoticed. However, if the cells are in a critical location – like the brain – then the consequences can be serious (or worse).
When you get a cold the virus replicates in a few thousand cells of your upper respiratory tract. The symptoms – headache, runny nose, craving for chocolate – are due to the immune response, not due to the direct cellular damage.
A bee is about 1/375,000th the weight of a human which, pro rata, means they have about 80 million cells. Still a lot of cells. Again, the loss of a few (or few thousand) due to virus replication might not be a problem.
Which is fortunate as some viruses are always present …
Deformed wing virus
Unless you live in Australia, where it’s not been detected, your bees are infected with deformed wing virus (DWV) {{3}}. In a hive with well-managed Varroa levels – either by the bees or the beekeeper – DWV quietly and unobtrusively replicates, apparently causing no damage to the host bee.
Since all honey bees have DWV, even those without Varroa, it’s necessary to include the ’apparently’ in the previous sentence. We can’t be certain that the bees don’t feel rubbish {{4}} as it’s difficult to make a meaningful comparison with bees that lack DWV.
Let’s assume they are OK.
The virus replicates, it’s passed from bee to bee during trophallaxis, and vertically via eggs and sperm, from the queen/drone to the workers. Every bee carries a few thousand replicating DWV virus particles. They are spread about when the bee defecates or when a forager visits a plant to collect pollen or nectar.
Inevitably this means that DWV is widespread in the environment. After all, honey bees are widespread and they forage some distance away from their nest site (the hive).
Inevitably this also means that some ecologists and environmental scientists are concerned that the DWV left in the environment by honey bees may infect other bees, such as the 24 bumble bee or ~225 solitary bee species we have in the UK.
This is a perfectly reasonable concern.
However, just because DWV is present doesn’t mean it can infect other bees or, if it does, that it will replicate in the recipient or, if it does, that it will cause damage.
An evolutionary perspective
It’s worth remembering that the Apis group of bees (which now includes the honey bee, Apis mellifera) diverged from other bees about 100 million years ago.
That period of time is more than sufficient to have produced huge changes in appearance, anatomy and the intracellular environment upon which viruses depend for replication.
100 million years is older than the earliest primate-like mammal. The Old World monkeys (from which humans eventually evolved) split about 35 million years ago and the last common ancestor of humans and chimpanzees existed about 8-9 million years ago.
Just because they’re called bees doesn’t mean they look or behave the same … or that they all share the necessary intracellular features that will support virus replication.
Some viruses replicate in a wide range of host species – even evolutionarily divergent hosts – but others are very restricted in their host range.
Infectious?
So, having provided some evolutionary caveats, let me return to the three points I made above that are necessary for DWV (or other honey bee viruses) to be a threat to other bee species. These are:
- is the virus infectious and can it infect?
- can it replicate?
- does it cause damage?
The virus is infectious if it has the potential to replicate after introduction to a host. But can it infect the host in the first place? Semantics, but significant.
Let’s deal with whether virus in the environment is infectious … this is actually best answered using honey bees.
Maurizio Mazzei and colleagues demonstrated that infectious DWV could be detected on pollen from flowers visited by honey bees (Mazzei et al., 2014). They did this by isolating DWV from pollen and injecting it into honey bees.
The bees became infected (they could discriminate between the DWV already present and the injected virus genetically), confirming that the DWV in the environment is – or at least can be – infectious.
Replication and pathology (disease) in other species?
Many years ago two bumble bees were identified with deformed wings. Sensitive genetic analysis demonstrated that DWV could be detected in (or on?) these bees.
This was taken as proof that DWV causes deformed wings in bumble bees … 🙁 .
Er, not so fast!
Just because something is present does not mean that it’s the causative agent of the symptoms … I’ll return to this point later.
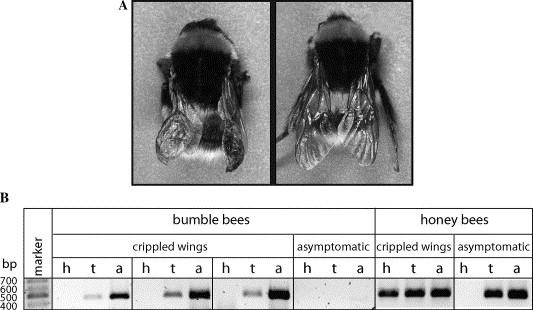
Subsequent studies have shown that, although DWV can replicate in bumble bees, there’s little evidence that the virus causes deformed wings.
Or for that matter any overt symptoms (Gusachenko et al., 2020).
I’ve discussed some of the studies that my lab published previously and so won’t rehash them in detail here.
Briefly, we either fed or injected bumble bees with DWV but could only show replication after injection. We even fed complete nests of bumble bees for a month – maintained in the laboratory – with huge amounts of DWV and could detect no virus in workers, the queen, brood or eggs.
Where’s the proof?
Had we just looked for the presence of DWV we would have found it, but we specifically looked for replicating virus by analysing injected/fed bees for the presence of an intermediate product in the replication cycle called a ‘negative strand’ (an inverted copy of the virus genome used as a template to make more copies, a little like the inked plate in an old printing press).
DWV does replicate in bumble bees but I’ve yet to be convinced that DWV is a threat to bumble bee health. Our analysis was relatively crude, but we did not detect any overt pathology … though I acknowledge they may have felt rubbish and had a craving for chocolate. More work is needed here.
In fact, the same really applies to honey bees. In the absence of a syringe (Varroa) DWV appears to do little or no damage to honey bees.
Osmia bicornis
Bumble bees get a lot of attention as they’re big, relatively easy to study, widespread and used commercially (for pollination in glasshouses and poly tunnels). But there are hundreds of other bee species.
Does DWV infect these? Does it replicate and does it cause disease?
Osmia bicornis is the scientific name of the red mason bee. This is a solitary, hole nesting bee, widespread in England and mainland Europe. Its range is expanding and it is now present in the central belt of Scotland {{5}}, Fife, Angus and with a few records from further north.
Alexandria Schauer and colleagues have recently investigated replication of DWV in this solitary bee (Schauer et al., 2023). Previous studies had shown that DWV was detectable in Osmia (of two different species) but, as the authors note:
‘the mere detection of a virus is nonsynonymous with actual replication within its host; it may rather reflect that an individual has ingested or carries viral particles that are not actively replicating.’
They therefore looked for the presence of the negative strand of the virus, something that only exists when the virus is replicating and that is not present within the virus particle.
They tested infection after micro-injection. Whilst not necessarily being a relevant method of transmission ‘in the field’ it is direct and bypasses a slew of innate gut protection mechanisms that might prevent infection … remember, the important question was does the virus replicate (and so potentially cause disease) in Osmia?
DWV replication in the red mason bee
The long and the short of it is that DWV does not replicate in the red mason bee.
Injected bees survived as well as the controls and the authors were unable to detect the negative strand ‘smoking gun’ that confirms replication.
However, in an interesting twist to the story, Schauer and colleagues also extracted viruses from the red mason bees 16 days after they had been injected with DWV.
They then injected this virus preparation into honey bee pupae and demonstrated that the DWV extracted from the Osmia was still infectious for honey bees.
Wait a second … aren’t the honey bees already infected with DWV?
Well done, you’re paying attention 🙂 .
The DWV originally injected into the Osmia was genetically distinct from the DWV already present in the honey pupae they subsequently injected. This allowed the authors to determine that the Osmia-extracted DWV remained infectious.
This is interesting.
It raises the possibility that bees such as Osmia, although not themselves replicating DWV, could act as an intermediate and transmit the virus back to honey bees, or – potentially – to other bees in the environment.
Possibility is the key point here … not certainty. It’s difficult to envisage a mechanism by which Osmia could transmit the virus to another host.
Predation might be one route I suppose?
Since the virus is not replicating in Osmia the amount of virus will never be higher than that inoculated which – in the case of environmental exposure – means vanishingly little.
It’s worth noting before I move on that there is one report of DWV replicating in another solitary bee, Andrena haemorrhoa, which is a species of mining bee (Radzevičiūtė et al., 2017).
OK, enough about a virus you already know about … here’s one that’s new.
The new kid on the block
A very recent study has identified a new honey bee virus, AmSV1, an entirely understandable abbreviation for Apis mellifera solinvivirus type 1. The work is published in the journal Viruses and is freely available (Ryabov et al., 2023 {{6}} ).
The virus was isolated from worker bees in a commercial apiary in Oregon that had experienced an ’historically high level of colony losses’.
Recent advances in nucleic acid sequencing and computation biology mean that a metagenomic approach can be used to identify novel viruses. Metagenomic is a fancy word meaning that the scientists characterise everything from the bee sample and eliminate everything that’s already known from subsequent analysis.
What’s left is the stuff that’s novel.
Of course, it’s a bit more complicated than that … the computational sieving (bioinformatics) allows lots of little pieces of the unknowns to fall through which can then be pieced together – again using computers – to enable them to be identified.
Clever 😉 .
AmSV1
Although AmSV1 is new, solinviviruses are not. Similar viruses have been identified in a range of invertebrates – beetles, aphids, the Asian honey bee, mosquitoes – and they are known to cause disease in fire ants and cultivated shrimp.
Virus particles were purified and the virus was injected into honey bee pupae. Using a similar approach to that described above, the negative strand replication product was detectable, unequivocally demonstrating that AmSV1 is able to replicate in honey bee pupae.
The fate of these pupae was not mentioned … did they emerge?
The distribution of the virus in adult bees was investigated by slicing and dicing naturally infected bees into three parts – head, thorax and abdomen – and testing each individually. Virus was found in all three parts, though not in every bee, suggesting it causes systemic infection.
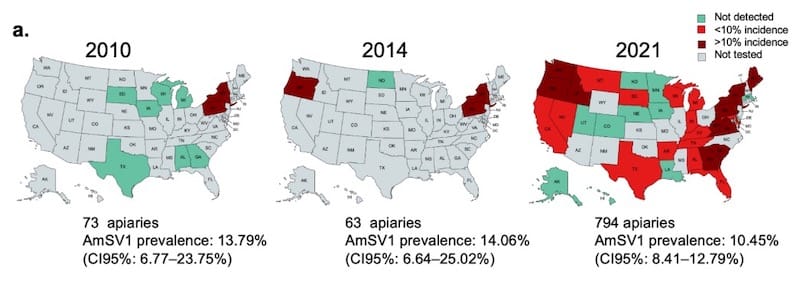
Having identified the virus it was then possible to develop molecular methods to detect how widespread the virus is in the USA and, using historical samples, whether it is increasing in prevalence and/or spreading geographically.
The historical samples were limited in number (and 2010 samples did not include Oregon), but it certainly appears that AmSV1 is now widespread in the USA and that it is spreading (note for example, AmSV1 was not detected in Texas, Alabama or Georgia in 2010, but that it was present in 2021).
Over the calendar year, AmSV1 was much more frequently detected in summer months – June and July – though the number of winter samples was very limited and so may be misleading.
Finally, the authors indirectly surveyed AmSV1 distribution ‘globally’ by computationally screening databases of honey bee sequences from Brazil, China, Turkey and Europe (UK and Germany).
AmSV1 was not detectable outside the USA.
AmSV1 and queens
About 50% of the readers of this site are in the USA.
AmSV1 is probably already in your state or, if it’s not there already, it soon will be.
The apiary-level sampling (i.e. pooled samples from 7-8 colonies from a single apiary) that contributed to the map above also involved recording the levels of some known pathogens – Nosema, Varroa, DWV, CBPV etc. – together with other problems that sometimes afflict honey bee colonies. These include small hive beetle, EFB, drone laying queens, the presence of queen cells, queenlessness or wax moths.
It was then possible, using odds ratio analysis, to determine if the presence of AmSV1 correlated with the presence of any of these known pathogens or field apiary observations.
There was no association with Varroa, suggesting the virus may not be mite-transmissible {{7}}.
Interestingly, the only field observation that was statistically relevant when linked to the presence of AmSV1 was queenlessness.
Apiaries with detectable levels of AmSV1 were nearly twice as likely to contain queenless colonies.
Does AmSV1 cause queen losses? Or does the absence of a queen, or queen problems, make the colony more susceptible to AmSV1 infection?
It’s perhaps notable that solinvivirus infection of fire ants reduces the fecundity (egg laying rate) of queens, resulting in lower levels of brood and workers.
The paper concludes by stating that solinviviruses are poorly understood and that more research is needed. As an ex-virologist I couldn’t agree more … but as an ex-academic I’m also aware of some of omissions from the paper which surprised me.
Omissions
All scientific papers have omissions.
Stuff is omitted because it’s going to be included in a follow-up paper that is already in preparation. Hence, the ’minimal publishable unit’.
It’s omitted because the results are – frankly – unconvincing and won’t stand close scrutiny by the peer reviewers. If they don’t like that bit perhaps they’ll reject the manuscript?
They’re omitted because they simply don’t fit the story. The results are good but their inclusion will cause confusion – the researcher doesn’t understand how they fit, so how on earth will the reader cope?
Or, of course, they’re omitted because they don’t actually exist … the reader (me, in this case) thinks they should exist because I’d have wanted the information before I wrote the paper 😉 .
What happened to those pupae injected with AmSV1?
If you incubate larvae in the lab they pupate and eventually emerge as adult workers. If AmSV1 was highly pathogenic to pupae you would easily be able to determine this. The virus was present in adult bees (in apiary sampling) so I suspect it is not highly pathogenic to pupae … or that it only infects bees as adults.
What was the cause of the ’historically high level of colony losses’ in the Oregon apiary?
This is a commercial beekeeping operation. Surely they would know if their colonies were queenless? Were the sampled colonies actually queenless? Were colonies lost in the summer – when AmAV1 levels are high – or winter?
My suspicion is that AmSV1 was not the cause of those colony losses. There’s an ‘in preparation’ paper from the same authors cited (reference 10) which hints at a different explanation …
Apiary-level virus complexes rather than specific viruses are associated with increased colony mortality in underperforming beekeeping operations. {{8}}
Which I’ll cover when it finally appears (if it’s interesting enough).
So, should you be worried?
No. There are lots of viruses out there that are yet to be detected. Literally thousands of them. Large scale sequencing projects have given us an inkling of just how little we know about the range and diversity of viruses that infect animals (Harvey and Holmes, 2022).
The viruses scientists first identified were the easy ones to isolate, usually associated with disease in humans or the animals we rely upon for food (foot and mouth disease virus of cattle was the first animal virus to be discovered, in 1898).
But we’re now realising that there are almost limitless numbers of other viruses in addition to the major pathogens already detected.
I’m not aware of a single living species that does not have one or more viruses that infects it.
There are even viruses of viruses.
Some of these yet-to-be-discovered viruses will undoubtedly be major pathogens but many, and probably most, won’t be.
In the absence of an obvious and unequivocal association between AmSV1 and honey bee disease (within which I’m including queen losses) I’d instead concentrate upon the viruses we already know cause problems if you want to worry about anything.
That association currently does not exist … so don’t worry about it.
The majority of colony losses occur in the winter, and the majority of those can usually be attributed to the ’dastardly duo’ of DWV and Varroa. Once those are under control everything gets a lot easier.
References
Harvey, E., and Holmes, E.C. (2022) Diversity and evolution of the animal virome. Nat Rev Microbiol 20: 321–334 https://www.nature.com/articles/s41579-021-00665-x.
Gusachenko, O.N., Woodford, L., Balbirnie-Cumming, K., Ryabov, E.V., and Evans, D.J. (2020) Evidence for and against deformed wing virus spillover from honey bees to bumble bees: a reverse genetic analysis. Sci Rep 10: 16847 https://www.nature.com/articles/s41598-020-73809-3.
Mazzei, M., Carrozza, M.L., Luisi, E., Forzan, M., Giusti, M., Sagona, S., et al. (2014) Infectivity of DWV Associated to Flower Pollen: Experimental Evidence of a Horizontal Transmission Route. PLOS ONE 9: e113448 https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0113448.
Radzevičiūtė, R., Theodorou, P., Husemann, M., Japoshvili, G., Kirkitadze, G., Zhusupbaeva, A., and Paxton, R.J. (2017) Replication of honey bee-associated RNA viruses across multiple bee species in apple orchards of Georgia, Germany and Kyrgyzstan. Journal of Invertebrate Pathology 146: 14–23 https://www.sciencedirect.com/science/article/pii/S0022201116302622.
Ryabov, E.V., Nearman, A.J., Nessa, A., Grubbs, K., Sallmann, B., Fahey, R., et al. (2023) Apis mellifera Solinvivirus-1, a Novel Honey Bee Virus That Remained Undetected for over a Decade, Is Widespread in the USA. Viruses 15: 1597 https://www.mdpi.com/1999-4915/15/7/1597.
Schauer, A., Bianco, N., Yañez, O., Brown, A., Albrecht, M., and Neumann, P. (2023) Deformed wing virus prevalence in solitary bees put to the test: an experimental transmission study. Frontiers in Ecology and Evolution 11 https://www.frontiersin.org/articles/10.3389/fevo.2023.1122304.
{{1}}: Meaning ‘this has to be such’.
{{2}}: About … the actual number is unknown.
{{3}}: And even if you live in Australia they may be …
{{4}}: Or have a craving for chocolate.
{{5}}: Edinburgh and Glasgow.
{{6}}: A lifetime ago the lead author, Eugene Ryabov, worked with me in the University of Warwick.
{{7}}: Or, perhaps, that the ubiquity of Varroa, coupled with its fluctuating population over the season due to brood levels or miticide application, might render this comparison meaningless.
{{8}}: My bold text.







Join the discussion ...