Droning on
This post was supposed to be about Varroa resistance in Apis mellifera – to follow the somewhat controversial ‘Leave and let die’ from a fortnight ago. However, pesky work commitments have prevented me doing it justice so it will have to wait for a future date.
Instead I’m going to pose some questions (and provide some partial answers) on overwintering mites and the use of drone brood culling to help minimise mite levels early in the season.
Imagine the scenario
A poorly managed colony goes into the winter with very high mite levels. Let’s assume the beekeeper failed to apply a late summer/early autumn treatment early enough and then ignored the advice to treat again in midwinter when the colony is broodless.
Tut, tut …
The queen is laying fewer and fewer eggs as the days shorten and the temperature drops. There are decreasing amounts of the critical 5th instar larvae that the mite must infest to reproduce.
At some point the colony may actually be broodless.
What happens to the mites?
Do they just hang around as phoretic mites waiting for the queen to start laying again?
Presumably, because there is nowhere else they can go … but …
What about the need for nurses?
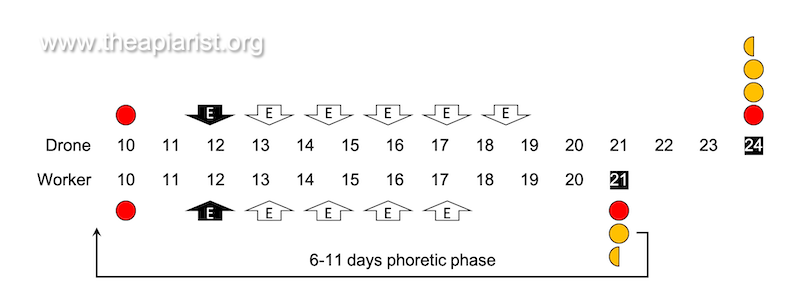
During the Varroa reproductive cycle newly emerged mites preferentially associate with nurse bees for ~6 days (usually quoted as 4-11 days) before infesting a new 5th instar larva.
Mites that associate with newly emerged bees or bees older than nurse bees exhibit reduced fecundity and fitness i.e. they produce fewer progeny and fewer mature progeny {{1}} per infested cell.
I’m not aware of studies showing the influence of the physiologically-distinct winter bees on mite fecundity.
Similarly, I’m not sure if there are any studies that have looked at the types of bees phoretic mites associate with during the winter {{2}}, or the numbers of bees in the colony during November to January {{3}} that might be considered to be similar physiologically to nurse bees.
Whilst we (or at least I) don’t know the answer to these questions, I’m willing to bet – for reasons to be elaborated upon below – that during the winter the fecundity and fitness of mites decreases significantly.
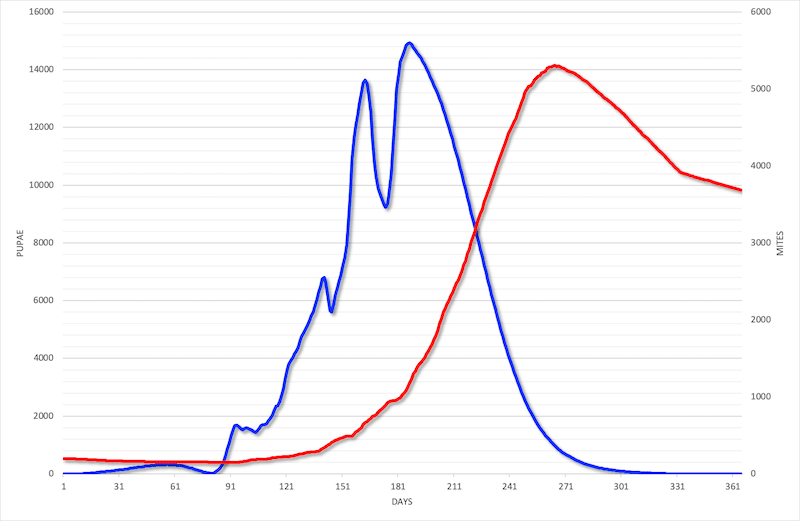
And the number of the little blighters …
Mite longevity
How long does a mite live?
The usual figure quoted for adult female mites is 2-3 reproductive cycles (of ~17 days and ~11 days for the first and subsequent rounds respectively). So perhaps about 40 days in total.
But, in the absence of brood (or if brood is in very short supply) this is probably longer as there is data linking longevity to the number of completed reproductive cycles i.e. if there is no reproduction the mite can live longer.
It is therefore perhaps reasonable to assume that mites should be able to survive through a broodless period of several weeks during midwinter. However, remember that this increases the chance the mite will be removed by grooming or other physical contacts within the cluster, so reducing the overall population.
Spring has sprung
So, going back to the scenario we started with …
What happens in late winter/early spring when the queen starts laying again?
Does that 5cm patch of early worker brood get immediately inundated with hundreds of mites?
If so, the consequences for the early brood are dire. High levels of mite infestation inevitably mean exposure to a large amount of deformed wing virus (DWV) which likely will result in precisely the developmental deformities you’d expect … DWV really “does what it says on the tin”.
My hives are carefully managed to minimise mite levels. I don’t really have any personal experience to help answer the question. However, in colonies that have higher (or even high) mite levels I don’t think it’s usual to see significant numbers of damaged bees in the very earliest possible inspections of the season {{4}}.
My (un)informed guess …
My guess is that several things probably happen to effectively reduce exposure of this earliest brood to Varroa:
- Varroa levels in the colony drop due to the extended winter phoretic phase. More opportunities for grooming or similar physical contact (perhaps even clustering) increase the loss of mites.
- Mites that remain may have reduced access to brood simply due to the mathematical chance of the bee they are phoretic on coming into contact with the very small numbers of late stage larvae in the colony.
- Mites that do infest brood have reduced fecundity and fitness and may not rear (m)any progeny.
There are a lot of assumptions and guesswork there. Some of these things may be known but discussions I’ve had with some of the leading Varroa researchers suggest that there are still big gaps in our knowledge.
OK, enough droning on, what about drones?
Back to the imagined scenario.
What happens next?
Well, perhaps not next, but soon?
The colony continues to contract (because the daily loss of aged workers still outnumbers the daily gain of new bees) but the laying rate of the queen gradually increases from a few tens, to hundreds to a couple of thousand eggs per day.
And the colony starts to really expand.
And so do the mite numbers …
And at some point, depending upon the expansion rate, the climate and (probably) a host of factors I’ve not thought of or are not known, the colony begins to make early swarm preparations by starting to rear drones.
Drones take 24 days to develop from the egg and a further 12-16 days to reach sexual maturity. If the swarming period starts in the first fortnight of May, the drones that take part were laid as eggs in late March.
And drone larvae are very attractive to Varroa.
9 out of 10 mites prefer drones
Varroa replicates ‘better’ in association with drone pupae. By better I mean that more progeny are produced from each infested cell. This is because the drone replication cycle is longer than that of worker brood.
On average 2.2 new mites are produced in drone cells vs only 1.3 in worker cells {{5}}. From an evolutionary standpoint this is a significant selective pressure and it’s therefore unsurprising that Varroa have evolved to preferentially infest drone brood.
Irrespective of the mite levels, given the choice between worker and drone, Varroa will infest drone brood at 8-11 times the level of worker brood {{6}}.
Significantly, as the amount of drone brood was reduced (typically it’s 5-15% of comb in the hive) the drone cell preference increased by ~50% {{7}}.
I hope you can see where this is now going …
Early drone brood sacrifice
As colony expansion segues into swarm preparation the queen lays small amounts of drone brood. These cells are a very small proportion of the overall brood in the colony but are disproportionately favoured by the mite population.
And the mite population – even in a poorly managed colony – should be less (and less fit) in the Spring than the preceding autumn for reasons elaborated upon above (with the caveat that some of that was informed guesswork).
Therefore, if you make sure you remove the earliest capped drone brood you should also remove a significant proportion of the viable mites in the colony.
Drone brood is usually around the periphery of the brood nest, along the bottom of frames with normal worker foundation, or on the ‘shoulders’ near the lugs. The drone brood is often scattered around the brood nest.
As a consequence, if you want to remove all the earliest capped drone brood you have to rummage through the frames and ‘fork out’ {{8}} little patches here and there.
It can be a bit of a mess.
Is there an easier way to do this?
Beekeepers who predominantly use foundationless frames will be aware that they usually have significantly more drones (and drone comb) in their colonies than equivalent sized colonies using embossed worker foundation.
Depending upon the type of foundationless frames used the drone comb is drawn out in different positions on the frames.
Horizontally wired foundationless frames can be all drone brood or a mix of drone and worker. However, the demarcation between the brood types is often inconveniently located with regard to support wires.
In contrast, foundationless frames constructed using vertical bamboo supports are often built as ‘panels’ consisting entirely of drone or worker comb.
Which makes slicing out one or more complete panels of recently capped drone brood simplicity itself.
There are no wires in the way.
You can sometimes simply pull it off the starter strip.
Check the brood for Varroa {{9}}, feed the pupae to your chickens and/or melt out the wax in your steam wax extractor.
The bees will rapidly rebuild the comb and will not miss a few hundred drones.
They’ll be much healthier without the mites. Importantly, the mites will have been removed from the colony early in the season so preventing them going through repeated rounds of reproduction.
This is the final part of the ‘midseason mite management‘ triptych {{10}}, but I might return to the subject with some more thoughts in the future … for example, continuous culling of drone brood (in contrast to selective culling of the very earliest drone brood in the colony discussed here) is not a particularly effective way of suppressing mite levels in a colony.
{{1}}: These two things are subtly different.
{{2}}: You can find quotes on mites overwintering for two months between the sclerites of worker bees, but I’m unaware of the primary studies where these statements derive.
{{3}}: Remembering here the usually caveats that I’m writing about a temperate Northern hemisphere location.
{{4}}: I don’t mean mid-April here, I mean late February/early March … not that you should really be inspecting the colony at that time of year.
{{5}}: These are calculations at the lower end of the range – see Know your enemy for more details.
{{6}}: Fuchs (1990) Preference for drone brood cells by Varroa jacobsoni Oud in colonies of Apis mellifera carnica. Apidologie 23:193-199; Boot et al., (1995) Invasion of Varroa jacobsoni into drone brood cells of the honey bee, Apis mellifera. Apidologie 26:109-116.
{{7}}: From about 8:1 to 12:1.
{{8}}: One way to do this is with a barbaric drone brood uncapping fork, but you can also just scrape them out with the blade of the hive tool.
{{9}}: Is the infestation level so high that miticide treatment is needed?
{{10}}: Though it could hardly be considered ‘art’, so I’m using this term incorrectly.





Join the discussion ...