Is it time for oxalic acid?
Synopsis: Effective oxalic acid treatment requires little or no brood in colonies. These conditions will likely occur in the next few weeks. How can you easily determine brood levels in the winter?
Introduction
Many beekeepers will already be thinking about the season ahead. Planning plans and scheming schemes. I expect that a number of them (perhaps even you?) are planning to expand their colony numbers.
Whether they achieve this by simply splitting a colony and adding a purchased mated queen, or by rearing their own queens and making up nucs, you probably know that the chances of a successful outcome are increased if the colony you start with is strong.
I’d define success simply as ending up with more strong colonies.
If you split a weak colony there won’t be sufficient bees to thrive. They’ll struggle to build up, they won’t collect a surplus of honey and they might get robbed out by wasps in the autumn.
Strong colonies solve – or simply avoid – the majority of beekeeping problems. They are better able to look after themselves and a lot more productive.
You can split a nuc off from a strong colony, add a virgin queen and easily end up with two excellent colonies at the end of the season.
What’s more, the ‘parental’ colony should also produce a good crop of honey … and the fast-expanding nuc might as well.
If the bees could plan, they would also be intending to increase colony numbers next season. Success for a colony is measured both in survival from one year to the next and the production of swarms (reproduction).
Colonies that do not swarm are inevitably doomed; starvation, fire, disease, failed queen mating or a marauding bear will – sooner or later – result in their demise.
The colony
The size of the colony fluctuates during the year. You can measure it in a variety of ways – however let’s just focus on two of them for this post:
- the total number of adult bees, or
- the amount of brood (e.g. eggs, larvae or pupae) present
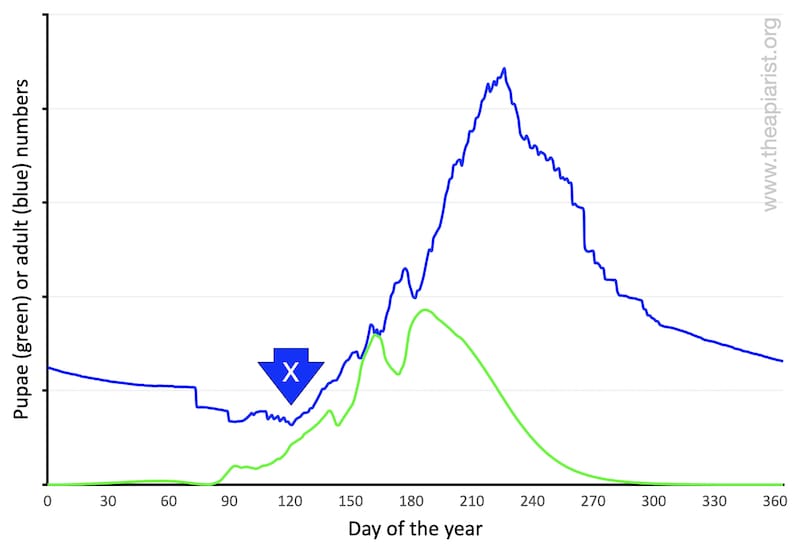
Colony size, in terms of adult workers (blue line, below), peaks sometime in early summer. Pupal number (green line) peak a little earlier. At this time of the season my hives (remember that my bees are reasonably dark native or semi-natives) probably have 10-11 frames of brood and an adult bee population of ~25,000.
Get used to this graph. It, or derivatives, will be appearing again.
If you run prolific Italian bees you’ll probably be thinking that 10 or 11 frames of brood isn’t particularly strong …
Yadda yadda, whatever.
… whether your bees are bright yellow or jet black, the colony size is smallest at some point in the winter and largest sometime in early summer.
The absolute numbers do not matter.
In the winter the colony consists of the long-lived (diutinus) winter bees (and the queen of course). These workers were reared late the previous summer and – assuming you minimised mite numbers and so reduced the levels of the longevity-destroying deformed wing virus (DWV) – will still mostly be present the following early spring.
Spring build up
The colony needs to expand from the (relatively small) winter colony size to the (booming) early summer population in order to be strong enough to swarm.
This process is often termed the ‘spring build up’ and involves three interrelated factors:
- weather
- forage
- bees
If the weather is lousy the bees will not be able to forage and build up will be retarded. However, even in good weather, if the environment lacks sufficient forage – spring nectars and pollen – build up will be delayed until it becomes available.
But integral to the entire process is the availability of bees to rear new brood. Rearing lots of brood requires lots of nurse bees.
It takes bees to make bees.
There’s no point in the queen laying up frame after frame of eggs if there are insufficient workers to keep the resulting brood fed and warm.
So the winter bees rear a small amount of brood, which emerges and rears a bit more, which rears a bit more, and … you get the idea.
As the old winter bees gradually die off they are replaced by new young workers. However, for much of the first month or two of the year (and perhaps most of March or even into April) the total adult bee population shrinks.
Some beekeepers term the date when colonies start to expand again as crossover day – marked with an X on the graph above.
Slow starters
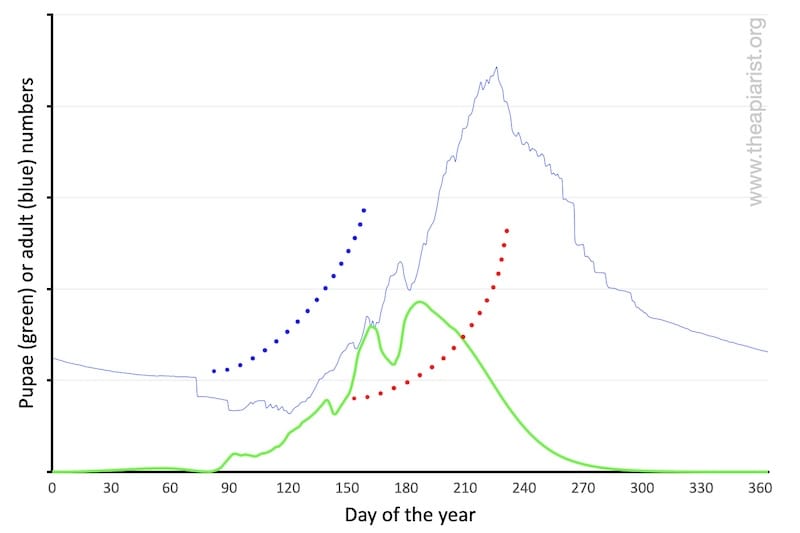
Brood rearing starts slowly and then increases; a few cells to start with, then a few dozen, half a palm’s worth on opposing frames, some lateral expansion onto the adjacent frames and so on.
If the bees start rearing brood too early (blue dotted line above) they risk running out of winter stores; colony expansion will stall if the weather is poor and they cannot forage. If the stores are exhausted, or foraging is impossible, the brood will be sacrificed or abandoned. The colony might starve in a cold spring.
However, if they start brood rearing too late (red dotted line above) the colony size will never expand sufficiently in time to swarm. Potentially the colony may not even expand enough to have the forager numbers needed to collect a honey surplus … survival over the following winter may be at risk.
A gradual start is a biological inevitability. It also means that relatively few ‘resources’ (food, effort, brood) are committed early in the season should conditions deteriorate.
Playing ‘catch up’
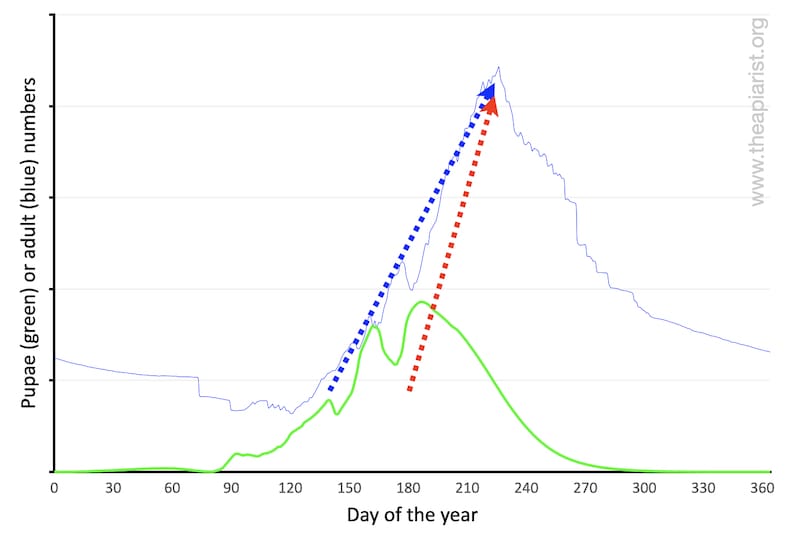
Why can’t they just start later and ‘catch up’ by rearing brood faster?
For example, if the start of the normal spring build up (shown with the blue dotted line below) was delayed, could the colony just rear more brood faster (shown with the red dotted line below)?
Probably not. The rate of colony expansion is limited by the number of bees available to rear brood and – critically – the 21 day development cycle of workers. This cannot be reduced … it’s invariant.
Literally, a rate limiting step.
So, at some point during the winter the bees need to start rearing the first brood that will allow a timely spring build up.
And the time they do this is in the next few weeks.
And before that they will be broodless.
Perhaps.
Never stopping
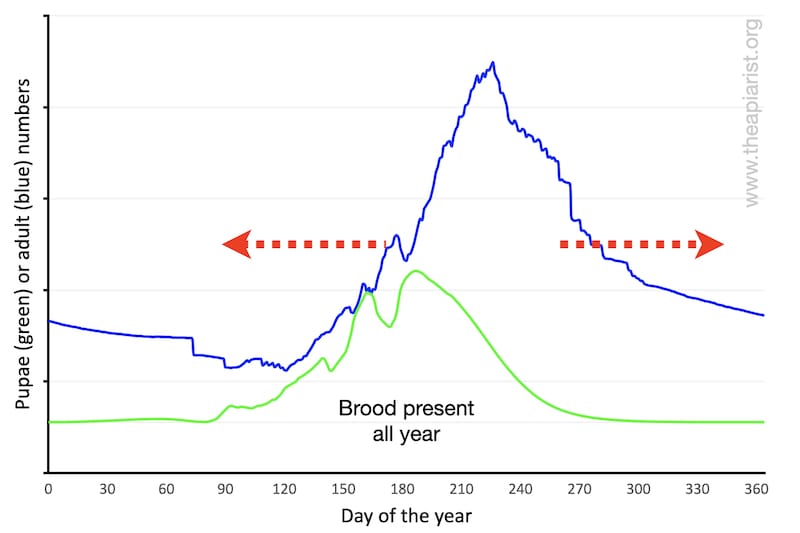
Depending upon your location, your bees may never be broodless in the winter. However, there will be a period in the winter when brood levels are low or very low.
The overall shape of the pupal population is the same as previously, it’s just that the baseline is raised.
If they rear brood throughout the winter the ‘area under the line’ will be increased. Warmer springs and autumns – a likely prerequisite – will mean the hive is more populous for a longer period of the season. I couldn’t be bothered to redraw the graph, so simply indicate this with a couple of red arrows.
However, although never broodless, there will still be a period when brood levels in the colony are minimal.
Why am I labouring this point?
The timing of the initiation of spring expansion is critical for the reasons outlined above. This expansion follows a period when the level of brood is minimal or – if you’re fortunate – zero.
‘Fortunate’ because the less sealed brood there is in the colony, the greater the proportion of Varroa mites will be phoretic, piggybacking around on the bodies of young bees and – importantly – accessible to oxalic acid.
Oxalic acid (OA) is only effective against phoretic mites. It does not penetrate brood cappings and – after a single administration (which is what is both recommended and approved) – it only remains active for a very few days.
Therefore, if you intend to treat with OA, it is really important to try and identify the period where brood levels in the colony are at an absolute minimum.
Ideally zero.
When is the colony broodless?
Unfortunately, you cannot easily predict when this ‘minimum brood’ period occurs.
Well, I can’t, at least not accurately.
Its timing is influenced by a number of things, such as:
- Age of the queen. Young queens lay much later into the autumn whereas old queens shut up shop relatively early. Do they also start earlier?
- Ambient temperatures. If foraging is impossible due to the weather – rain or low temperatures – or futile because there is no forage then the colony is less likely to rear brood. If the temperature is so cold the colony clusters then brood rearing is reduced.
- Photoperiod. This is a guess, but I wouldn’t be surprised if shorter late autumn days reduced brood rearing, though separating the effects of temperature or other environmental factors might be tricky.
- Colony strength? Pure speculation. How does colony strength influence the termination and restarting of brood rearing?
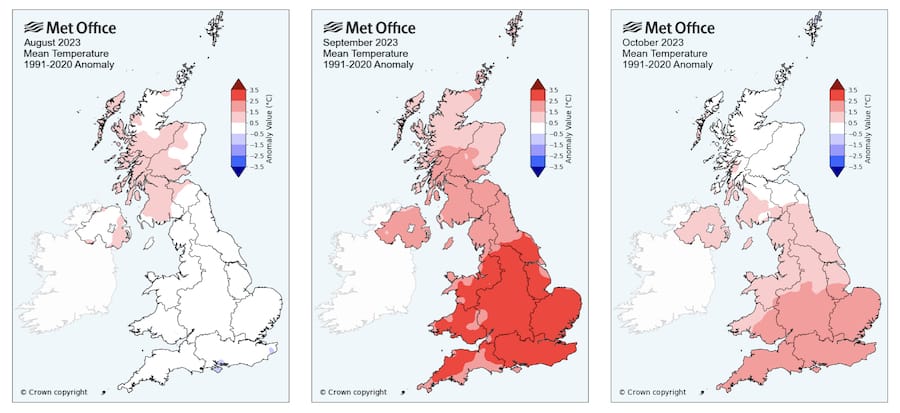
Autumn this year (2023) has been unusually warm.
August was pretty typical over much of the country, but September and October – particularly in southern England – had mean temperatures 1.5° – 2.5°C warmer than a 30 year average. I expect that this means colonies will have reared brood later into the autumn.
In previous (colder) years some of my colonies have been broodless in late October.
November is now reverting to type. In my apiaries temperature maxima are now 8-9°C and there are occasional frosts.
I’ve opened several colonies recently and they are all more or less clustered. I’ve not looked for brood … at least not directly. However, I have added trays underneath the open mesh floors to look for evidence of brood rearing indirectly.
Go with the evidence …
If the colony is rearing brood there will be eggs, larvae and sealed pupae in the hive. It’s only really the sealed brood you need to be concerned about in terms of ‘hidden’ mites.
To emerge from capped cells the fully developed workers have to chew through the biscuit-coloured pollen-impregnated wax cappings.
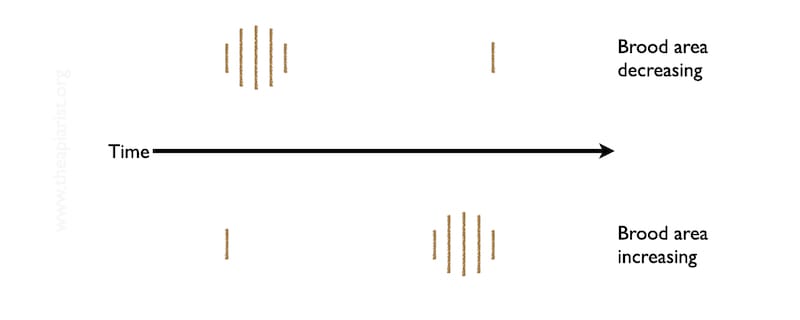
These cappings fall through the cluster of bees, through the open mesh floor and onto the white Correx tray I’ve placed underneath the floor.
They appear as a series of fuzzy stripes, directly underneath the parallel seams of bees.
The more stripes there are, and the wider those stripes are, the more brood is emerging.
Leave the tray in place for 2-3 days and then check it. If there is evidence of brood cappings on the tray remove it, clean it and put it back in a week or so.
When you check a second time decide whether there are fewer and/or narrower stripes, or whether there are more and wider.
If there are no ‘cappings’ stripes the colony is broodless. Reducing numbers or size of the stripes means it should soon be broodless. If the stripes are stable or increasing the colony is continuing to rear brood.
With two or three checks at this time of the year you can get a pretty good idea of what’s happening inside the hive.
To achieve a timely spring build up, many colonies will be rearing brood by late December. Here in Scotland my colonies are almost all broodless by early/mid-November (and sometimes earlier), but they start again in December and have capped brood by the end of the month.
… the right evidence
I described brood cappings as ‘biscuit-coloured pollen-impregnated wax’. These crumbs are visible as fuzzy lines on the white Correx tray.
Don’t confuse these with the much paler coloured cappings – that lack pollen – from uncapped stores. These are often, though not exclusively, from the periphery of the brood nest.
Does this really matter?
Why go to all this trouble?
Why not do exactly what everyone else in your association does and trickle treat your colonies on the 30th of December?
It’s a Saturday. You should might have recovered from the excesses of Christmas and the fresh air will revitalise you before yet more overindulgence at Hogmanay.
Some associations even help their members treat colonies in the lull between Christmas and New year by distributing pre-made OA solution, saving everyone the (negligible) trouble of making their own up.
Surely that would be easier?
So, does it matter?
Yes … it does matter.
At least it matters if you want your OA treatment to be maximally effective. Why bother doing it unless that is your goal?
I consider chemical treatment for mites a ‘necessary evil’. I’d prefer not to have to do it but, since I do treat, I want to slaughter as many of the little blighters as possible. That means 90-95% of them.
And to achieve that I need to be able to get the oxalic acid to them.
90-95%
Oxalic acid is very effective – administered appropriately – against phoretic mites.
90-95% of phoretic mites are killed after OA exposure.
So what are the consequences of having a bit of sealed brood (in which a few mites are hiding) in the colony?
The more mites that are present in capped cells during OA treatment, the greater the number of mites that will survive to harm the colony in the following season.
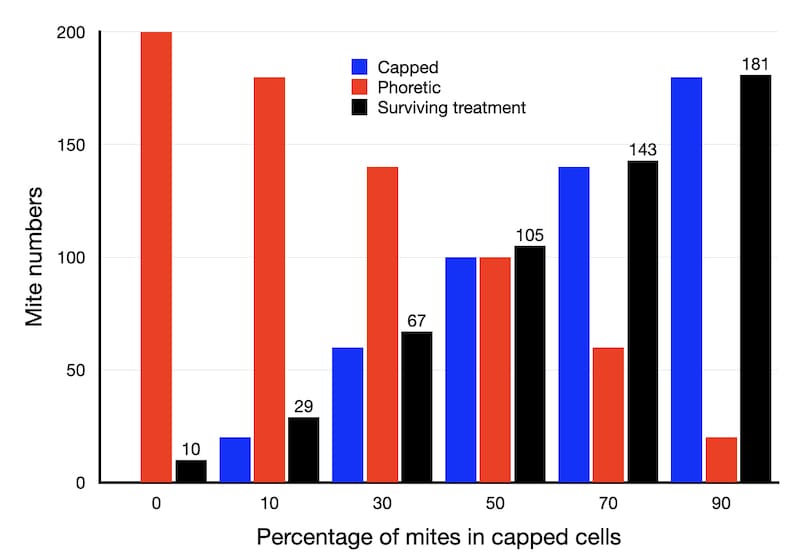
Let’s assume there are a total of 200 mites in the hive now.
Is that an unreasonable assumption?
Look again at the maps above; it’s been a very warm autumn.
Any mites surviving the summer miticide treatment will likely have been busy reproducing in the brood reared late into the unusually warm autumn weather we’ve had.
So … 200 mites. OK?
Here’s a graph showing the number of mites that survive OA treatment (the black bars) depending upon the percentage of those mites that are hiding in capped cells at the time of treatment. I’ve assumed an efficacy of 95% for OA treatment in these calculations.
If there is no sealed brood in the hive (all the mites are phoretic) then only 10 of the 200 mites will survive the treatment.
That’s a great start to the season ahead.
However, if 50% of the mites are in capped cells, 105 of the 200 will survive treatment.
Hmmm … not so good.
Finally, if there’s a lot of sealed brood and 90% of the mites are in capped cells (see below) then 181 of the 200 will survive.
You may as well not bother treating. The disturbance to the colony when dousing them in OA will probably do more damage to the bees than to the mites.
Future problems
The more mites present at the beginning of the year, the sooner the population of reproducing mites will exceed 1000. This is the number the National Bee Unit considers is the level at which treatment must be applied.
With 181 at the start of the year, mite levels will exceed 1000 by mid-May. Reducing mite numbers to 105 gets you to the beginning of June. Killing sufficient phoretic mites for only 67 to survive buys you another fortnight or so. If only 29 mites are present at the start of the year then the 1000 threshold isn’t exceeded until the beginning of July.
However, if you only have 10 mites present at the beginning of January the threshold is not exceeded until October … by which time you should have already applied the autumn treatment.
These calculations were done with Randy’s Varroa model on the web (a browser-based version of Randy Oliver’s Varroa model) run at the default settings except I use the ‘hard winter model’ which better reflects the UK climate.
How many mites are in capped cells in the winter?
I don’t know.
In the summer the figure is usually quoted as ~90% but I’m not aware of any studies that have properly measured this in the winter.
With limiting amounts of brood it’s likely that mites will take longer to find a cell containing a suitably aged larvae to infest. There may be reduced mobility of bees within the cluster, further reducing mite access to larvae.
I would expect that the percentage of mites in capped cells – all other things being equal – would be (broadly) directly proportional to the amount of brood in the winter colony.
The more brood, the fewer phoretic mites … another compelling reason to treat when the colony is as close to broodless as possible.
One thing I am certain of … if there’s no sealed brood then all the mites will be phoretic.
Trickle or vape?
Trickle.
Oh, you want to know why?
For most beekeepers I think trickle treating with oxalic acid is the most sensible option. I think it makes particular sense when you only have a few hives to treat.
Firstly it means you don’t need to purchase an OA vaporiser (£30 to £300+), a power supply and the the associated PPE needed to use it safely.
Secondly, trickle treating is faster. Don’t believe the vapaholics who claim 30 seconds per hive. This figure assumes an expensive ‘active’ vaporiser (like a Sublimox) and ignores sealing the hive properly … and waiting sufficient time (~5 minutes, until all the vapour has settled) before unsealing it afterwards.
I have a Sublimox and I’ve done timed comparisons of vaping and trickling and the latter is faster (Yes, I really need to get out more).
Finally, the approved, commercially available OA powder (Api-Bioxal) contains glucose which caramelises and makes an unholy mess of a vaporiser.
There are two conditions when OA vaporisation may be preferable to trickle treating:
- When you have many adjacent hives (10+) to treat and you can seal/unseal them all at once. An assistant helps.
- If there’s open brood in the hive. The acidity of OA damages (i.e. kills) larvae and studies from LASI in Sussex (Al Toufailia et al., 2015) showed that trickle treated colonies with open brood were weaker in spring.
And, before anyone asks … I’ve covered repeated OA treatment previously. It’s not allowed in the UK and I’ve yet to see any compelling evidence that it is as effective as trickle treating a broodless colony in the winter.
I don’t make the rules … but I also only promote methods I know are effective.
Notes
The graphs of pupal and adult bee numbers were modelled using BEEHAVE (Becher et al., 2014) run with the default settings for latitude and weather (Rothamstead).
References
Al Toufailia, H., Scandian, L., and Ratnieks, F.L.W. (2015) Towards integrated control of varroa: 2)comparing application methods and doses of oxalic acid on the mortality of phoretic Varroa destructor mites and their honey bee hosts. Journal of Apicultural Research 54: 108–120 https://doi.org/10.1080/00218839.2015.1106777.
Becher, M.A., Grimm, V., Thorbek, P., Horn, J., Kennedy, P.J., and Osborne, J.L. (2014) BEEHAVE: a systems model of honeybee colony dynamics and foraging to explore multifactorial causes of colony failure. Journal of Applied Ecology 51: 470–482 https://onlinelibrary.wiley.com/doi/abs/10.1111/1365-2664.12222.




Join the discussion ...